My Baby's Movements in Womb Make Me Vomit
- Research commodity
- Open up Admission
- Published:
Vomiting in pregnancy is associated with a college risk of low nativity weight: a cohort written report
BMC Pregnancy and Childbirth volume 18, Article number:133 (2018) Cite this article
Abstract
Background
Low nascency weight has important short- and long-term wellness implications. Previously it has been shown that pregnancies affected past hyperemesis gravidarum in the female parent are at higher adventure of having low birth weight offspring. In this written report we tested whether such risks are also evident with less astringent nausea and vomiting in pregnancy.
Methods
Ane k two hundred 30-eight women in the prospective Cambridge Baby Growth Report filled in pregnancy questionnaires which included questions relating to agin effects of pregnancy and drugs taken during that time. Ordinal logistic regression models, adjusted for parity, ethnicity, marital and smoking status were used to relate the risk of giving birth to depression birth weight (< two.5 kg) babies to nausea and/or vomiting in pregnancy that were not treated with anti-emetics and did not report suffering from hyperemesis gravidarum.
Results
Only three women in the cohort reported having had hyperemesis gravidarum although a further 17 women reported taking anti-emetics during pregnancy. Of those 1218 women who did not accept anti-emetics 286 (23.5%) did not feel nausea or vomiting, 467 (38.three%) experienced nausea simply non vomiting and 465 experienced airsickness (38.2%). Vomiting during pregnancy was associated with higher gamble of having a depression birth weight baby (odds ratio three.5 (1.2, 10.8), p = 0.03). The risk associated with airsickness was plant in the offset (p = 0.01) and 2nd (p = 0.01) trimesters but not the tertiary (p = 1.0). The higher risk was non evident in those women who only experienced nausea (odds ratio 1.0 (0.3, 4.0), p = one.0).
Conclusions
Airsickness in early pregnancy, even when non perceived to be sufficiently severe to merit treatment, is associated with a college risk of delivering a depression birth weight babe. Early pregnancy airsickness might therefore exist usable as a mark of higher adventure of low birth weight in pregnancy. This may exist of benefit in situations where routine ultrasound is not available to distinguish prematurity from fetal growth restriction, and then low birth weight is used as an alternative.
Background
Low birth weight (LBW) leads to a higher run a risk of perinatal mortality and morbidity, including impaired growth and cognitive development [i]. More long-term complications include college risks for high claret pressure [2] and cardiovascular disease [three], dumb glucose tolerance and type 2 diabetes [4], early age at menarche [5] and menopause [vi], and reduced os mineral density [7] and osteoporosis [viii]. LBW tin relate to 1 or both of premature nascency and fetal growth restriction, or being constitutionally minor, and risk of LBW tin can be related to such factors as ethnic differences, multiple nativity pregnancies, maternal age at birth, fetal environmental factors such as exposure to alcohol, smoking or illicit drugs, maternal nutrition during pregnancy, poor socioeconomic condition [ix] and genetic defects [ten]. Another risk factor appears to be hyperemesis gravidarum [11,12,13], a severe form of nausea and vomiting in pregnancy that can lead to maternal dehydration and weight loss. Treatment of severe nausea and vomiting in pregnancy with anti-emetics may even be associated with a reduction in the prevalence of LBW [14, fifteen], although such findings are by no means universal [16,17,18].
Whilst the association between hyperemesis gravidarum and college take a chance of LBW is reasonably well established, what is not so clear is whether potentially less severe nausea and/or vomiting in pregnancy is as well associated with the take a chance of delivering LBW babies. The but contempo related prove suggests that it may be associated with being small for gestational age (SGA) due to fetal growth restriction, one of the master reasons for a infant having a LBW [19]. This study was therefore designed to exam the hypothesis that nausea and vomiting in pregnancy, of insufficient severity to require handling, is associated with the risk of delivering a LBW babe. To exercise this we used data collected for the Cambridge Baby Growth Study.
Methods
Cohort
The prospective and longitudinal Cambridge Baby Growth Study recruited 2229 mothers (and their partners and offspring) attending ultrasound clinics during early pregnancy at the Rosie Maternity Hospital, Cambridge, United Kingdom, betwixt 2001 and 2009 [twenty]. All mothers were over 16 years of age. Birth weights of their babies, their sex and their gestational age at nativity were extracted from hospital notes, having been recorded there by midwives. LBW was defined every bit a birth weight of less than ii.5 kg. SGA was classified as being in the lowest tenth percentile for gestational historic period. Prematurity was defined equally existence born prior to week 37 of gestation. Categorisation according to whether or non the participants adult gestational diabetes [21] or gestational hypertension [22] has been described previously. In this cohort, 96.ix% of the offspring were of white ethnicity, 0.8% were of mixed race, 0.six% were black (African or Caribbean), 0.8% were East-Asian, and 0.9% were Indo-Asian.
Each of the study participants was given a printed questionnaire at recruitment with questions to answer and return once the pregnancy was completed [23]. They were encouraged to fill it in as the pregnancy progressed. The questionnaires included boxes to tick if the participants had experienced nausea or had vomited during pregnancy. If either of these boxes were ticked there were further boxes to fill in concerning the timing (i.due east. week(s) of pregnancy) when the nausea or vomiting were experienced. A further question asked "Have y'all taken any medicine during this pregnancy?" Those women who responded in the affirmative were and so asked to complete a tabular array with the following headings: "Name", "Disease", "Daily Dose", "No. of Days" and "Gestational Week(s)". This means that nausea and airsickness prior to attending the booking clinic would have had to have been recalled over a maximum period of several weeks whereas nausea and airsickness subsequent to that could exist recorded equally the pregnancy progressed (requiring recollection over a much shorter flow of fourth dimension). A total of 1238 women (54.6%) filled in the questionnaires; those that did not were excluded from the present analysis. For 598 of the pregnancies where the female parent failed to return a filled-in questionnaire the baby's birth weight was also missing. The nativity weights of the remaining babies, adjusted for pre-pregnancy maternal BMI, gestational age at birth, parity and sexual practice, were non different between those that completed the questionnaire and those that did not (filled in questionnaire 3.482 (iii.456, 3.508) kg 5. did not make full in questionnaire 3.403 (3.310, 3.497) kg, p = 0.1), although the prevalence of LBW in the offspring of the women that returned the questionnaires was lower (filled in questionnaire 37/1218 v. did not fill in questionnaire 27/431, p = v × 10− 3). Of those women that filled in their questionnaires only 3 reported that they had hyperemesis gravidarum and a further 17 were treated with anti-emetics: cyclizine (7), promethazine (5), prochlorperazine (4), metoclopramide (two), domperidone (2), prednisolone (ii), chlorphenamine (1), ondansetron (1), chlorpromazine (ane) and unknown (ane). These 20 women were excluded from this specific analysis in lodge to test just those women who had a potentially milder phenotype. The cocky-reported timings of exposure to nausea and/or vomiting were divided into trimesters (beginning trimester being up to gestational week 12, 2d trimester being weeks 13 to 27 and third trimester beingness from calendar week 28 onwards).
Categorisation and calculations
We categorised each of the women into one of iii different groups: those who reported neither nausea nor vomiting (north = 286), those who reported nausea merely not vomiting (n = 467) and those who reported vomiting (n = 465, 432 of whom reported nausea and vomiting and 33 who reported vomiting without nausea). Those that reported vomiting without nausea had no evidence of concurrent urinary or breast infections, or bear witness of the vomiting occurring but in the final trimester of pregnancy. The trunk mass indexes (BMI) were calculated dividing the maternal weights prior to pregnancy by their heights squared.
Statistical analysis
Associations with LBW were adjusted for the following confounders: parity, marital and smoking statuses, and ethnicity [24]. Associations between nausea and vomiting and quantitative continuous variables (such as BMI and maternal age) were tested using linear regression models (adjusted for confounders where necessary). Associations with dichotomous variables (such equally LBW) were tested using ordinal logistic regression (adapted for confounders where necessary), the χ2-test or Fisher'due south exact exam as appropriate. Unless otherwise stated all other data are presented as means (95% confidence intervals (CI)). Statistical analyses were performed using Stata 13 (StataCorp LP, Higher Station, Texas, U.S.A.). P < 0.05 was considered statistically significant throughout.
Results
Maternal clinical characteristics
Table i shows the clinical characteristics of the different groups tested. Those 465 women who experienced airsickness during pregnancy (432 of whom also reported nausea) tended to be younger (p = 1.5 × x− 3) and slightly heavier/more obese (pre-pregnancy weight p = 0.02, pre-pregnancy BMI p = 0.01) than those women who did not experience nausea or vomiting in pregnancy. These differences in clinical characteristics from those women who did not experience nausea or vomiting, were non evident in those women who experienced nausea but not airsickness.
Associations between maternal exposure to nausea and/or vomiting in pregnancy and nascency characteristics of the baby
The delivery of LBW babies was more than common in those women who experienced vomiting in pregnancy (when adjusted for confounders) than in those women who did not experience airsickness or nausea (p = 0.03; Table 2). This relationship was still evident after farther adjustment was made for maternal BMI prior to pregnancy (OR iii.1 (one.0, 9.7), p = 0.048). In dissimilarity at that place was no college risk of LBW associated with experiencing nausea but not vomiting in pregnancy (p = 1.0).
Table 2 shows other birth characteristics of women according to their experience of nausea and airsickness in pregnancy. Despite the differences in risk of LBW, there was no credible departure in mean nascence weight, gestational age at nascence or prevalence of prematurity or SGA. Similarly there was no apparent deviation in the birth weight adjusted for pre-pregnancy maternal BMI, gestational age at birth, sex and parity. However there was a slightly college proportion of female person babies built-in to mothers who experienced vomiting during pregnancy. Figure i shows Kernel density estimation plots for unadjusted birth weight, gestational age at nativity and nascency weight for gestational age according to maternal exposure to nausea and airsickness in pregnancy. Small differences in the distributions of these were evident in each plot.
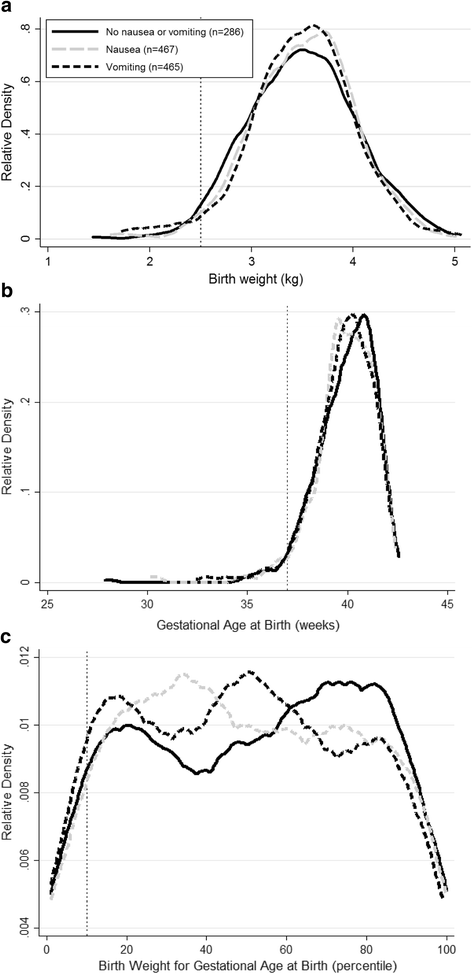
Kernel density estimation plots of a unadjusted nascency weight distributions, b gestational historic period at birth and c nascence weight adjusted for gestational age at nascency percentiles in the Cambridge Baby Growth Study in babies whose mothers were not treated with anti-emetics in pregnancy. The lines are plotted co-ordinate to the mother's exposure to nausea or vomiting in pregnancy. The cut off for LBW is shown by the dotted line at ii.v kg in (a), that for prematurity is shown past the dotted line at 37 weeks in (b) and that for SGA is shown past the dotted line at the tenth percentile of nativity weight adjusted for gestational historic period at birth in (c). Nausea refers to those women who experienced nausea merely not airsickness during pregnancy. Vomiting refers to those women who experienced airsickness during pregnancy, independently of whether or non they also experienced nausea
Associations between exposure to airsickness in different trimesters of pregnancy and birth characteristics of the baby
The college prevalence of giving birth to LBW babies in women who experienced airsickness was evident in those who experienced it in the first (OR 4.3 (one.4, 13.2), p = 0.01, n = 266 vomiting and north = 204 no nausea or vomiting) or second (OR 4.4 (1.iv, thirteen.nine), p = 0.01, n = 185 vomiting and due north = 204 no nausea or vomiting) trimester of pregnancy. Nevertheless information technology was not evident in those who experienced it in the tertiary trimester (OR ane.0 (1.0, 1.0), n = 36 vomiting and 204 no nausea or vomiting, p = 1.0).
Discussion
Airsickness in pregnancy, not treated with anti-emetics, is associated with a higher risk of giving nascency to LBW babies in this report. This is consistent with reported associations between LBW (or related phenotypes such as SGA) and hyperemesis gravidarum [eleven,12,13, 25,26,27,28], although such associations are not universal findings [29,xxx,31,32]. Nausea and airsickness with no reference to hyperemesis gravidarum has also been associated with a higher risk of LBW [33] and decreased birth weight [34] in some other studies, although no difference in risk was reported in others [19, 35,36,37]. Although a systematic review [38] reported a lower risk of LBW in association with nausea and vomiting in pregnancy the studies that it was based looked at anti-emetic utilise to categories study participants [14, 15]. This is therefore very different to our own study where nosotros specifically excluded women who took anti-emetics in case these drugs affected pathways involved in regulating LBW take a chance [39]. Similarly the very big Norwegian Female parent and Kid Cohort Study [40] constitute reduced population rates of LBW in association with nausea and airsickness in pregnancy, but did so without specifically excluding those women who took anti-emetics in pregnancy. Our study therefore presents results a slightly dissimilar population to those examined in other investigations, and one where we specifically investigated a potentially milder negative aspect of pregnancy than hyperemesis gravidarum.
This higher risk for LBW associated with vomiting, and nausea but just in the second trimester, appeared to be in the first two trimesters of pregnancy with an apparent lack of gamble in the third trimester. Studies are ongoing to effort and detect whether or non nausea and vomiting in pregnancy and resulting associations are genetically mediated [41, 42]. With an overall odds ratio of three.5 for LBW in our population, vomiting in early pregnancy may be a marker of chance for LBW that is useful for its prediction in conjunction with other risk factors. Where routine ultrasound is available small babies tend to exist assessed every bit either SGA (possibly due to fetal growth restriction) and/or premature rather than LBW, but the link with airsickness may be useful in areas where such scans are not available and LBW is used to encompass them both. Nausea and airsickness in pregnancy are thought to be protective towards the embryo/fetus in terms of reducing exposure to nutrient borne harmful substances such every bit infective microorganisms [43], and they can atomic number 82 to changes in the maternal dietary intake [44]. In that location is evidence that this can lead to positive furnishings on the fetus such as decreased rates of miscarriage and congenital malformations [45]. Whilst potentially being an advantage in a mild course, in excess it is possible that this airsickness might reduce nutrient delivery to the fetus leading to the greater risk of LBW [46]. The fact that in our population as a whole there was no credible subtract in mean birth weight despite the college prevalence of LBW suggests that whilst in that location is a negative impact of vomiting on nascency weight for some babies, in other babies a protective advantage may be evident [43]. The Kernel density estimation plot for birth weight in our population (Fig. i(a)) would appear to be consistent with this suggestion (as nascency weight density around the mean appeared to be college in those women afflicted by vomiting).
LBW often relates to the baby undergoing fetal growth brake and/or existence born prematurely. Given the increment in birth weight usually observed in male babies, in general at that place is too a slightly increased run a risk of LBW in female babies compared to males [ix]. In our population despite the association between vomiting and LBW, we did not find further significant independent associations between exposure to vomiting in pregnancy and the prevalence of SGA (a group that may take been enriched with babies who underwent fetal growth restriction) or premature births or the gestational historic period at birth. Looking at the Kernel density estimation plots for nascence weight in our population below the cut off for LBW the line for women who experienced vomiting was clearly a little higher than those representing the other groups (Fig. one(a)). The differences between the groups beneath the cut offs in the gestational ages at nascence (to assess the densities related to prematurity) and the nativity weights for gestational age (to appraise the densities related to SGA) plots were smaller though (Fig. ane (b) and (c)). Further studies are required to validate these findings in other cohorts, particularly those that are better able to distinguish whether the LBW relates more to SGA (and therefore probably fetal growth restriction), prematurity or a mix of the ii.
Whilst we could not ascribe the increased adventure of LBW to fetal growth restriction or premature birth we did annotation a slight excess of pregnant women conveying female babies. This backlog has previously been observed with hyperemesis gravidarum [47]. We recently reported that serum GDF-fifteen concentrations effectually week 15 of pregnancy were higher in those women reporting vomiting in the second trimester of pregnancy in the Cambridge Baby Growth Study [48], a hormone that may stimulate airsickness in pregnancy [49]. Interestingly we have as well observed an increased concentration of week xv serum GDF-xv concentrations in women conveying females compared to those carrying males in our population (Petry et al., unpublished ascertainment), which may explain the slight excess of pregnant women carrying females in the grouping that experienced vomiting.
The chief strengths of this study are its prospective nature and the fact that its design enabled us to study a grouping of women with a potentially milder, albeit still unpleasant phenotype than has been tested in other published studies. We have therefore uniquely been able to notice that the gamble of giving birth to a LBW baby is college with vomiting. The study's main limitation is that the nausea and airsickness and the taking of whatsoever anti-emetics to treat it were self-reported. The analysis could therefore have been afflicted by recall bias [50], although given that the women were encouraged to fill in their questionnaires as their pregnancies progressed rather than retrospectively the furnishings of this may have been limited. Indeed furnishings of any retrieve bias were clearly non sufficient to prevent u.s. from discovering our clan between increased circulating GDF-fifteen concentrations and 2nd trimester vomiting [48]. Another limitation of our written report may be a slight lack of statistical power to investigate potential effects of confounders. Nonetheless in that location was clearly sufficient power in our study to test associations between the maternal feel of nausea and vomiting in pregnancy and the risk of delivering LBW babies. The terminal primary limitation may be exclusion of women who took anti-emetics during pregnancy. This is because the threshold for nausea and vomiting where women sought and were and then prescribed anti-emetics would have varied from participant to participant, and therefore the exclusion could have been rather self-selecting. However the advantage of doing this is the lack of potential confounding in our written report of these drugs affecting pathways involved in regulating fetal growth, a strength of the study.
Conclusions
This report suggests that vomiting in early pregnancy of bereft severity to merit cocky-selected treatment with anti-emetics is associated with a higher risk of a adult female giving birth to a LBW baby. It is possible that this vomiting may therefore be a marker of LBW pregnancies. This could be useful in situations where routine ultrasound is non bachelor to distinguish prematurity from being SGA, so LBW is used equally an alternative to encompass them both.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- LBW:
-
Depression birth weight
- OR:
-
Odds ratio
- SGA:
-
Small for gestational historic period
References
-
Pallotto EK, Kilbride HW. Perinatal outcome and afterward implications of intrauterine growth brake. Clin Obstet Gynecol. 2006;49:257–69.
-
Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in developed life. BMJ. 1990;301:259–62.
-
Barker DJ, Wintertime PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and decease from ischaemic heart disease. Lancet. 1989;2:577–80.
-
Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Wintertime PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22.
-
Kirchengast S, Hartmann B. Association between maternal historic period at menarche and newborn size. Soc Biol. 2000;47:114–26.
-
Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early on age at natural menopause in a British nativity accomplice study. Hum Reprod. 2010;25:791–8.
-
Laitinen J, Kiukaanniemi K, Heikkinen J, Koiranen Thousand, Nieminen P, Sovio U, Keinänen-Kiukaanniemi Southward, Järvelin MR. Torso size from birth to machismo and os mineral content and density at 31 years of age: results from the northern Finland 1966 nativity cohort study. Osteoporos Int. 2005;16:1417–24.
-
Martínez-Mesa J, Restrepo-Méndez MC, González DA, Wehrmeister FC, Horta BL, Domingues MR, Menezes AM. Life-course evidence of nascency weight effects on bone mass: systematic review and meta-assay. Osteoporos Int. 2013;24:vii–18.
-
Valero De Bernabé J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martínez D, Domínguez-Rojas 5. Adventure factors for depression birth weight: a review. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15.
-
Yaghootkar H, Freathy RM. Genetic origins of depression nascency weight. Curr Opin Clin Nutr Metab Intendance. 2012;15:258–64.
-
Chin RK, Lao TT. Low nascency weight and hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 1988;28:179–83.
-
Dodds L, Barbarous DB, Joseph KS, Allen VM, Butler B. Outcomes of pregnancies complicated past hyperemesis gravidarum. Obstet Gynecol. 2006;107:285–92.
-
Veenendaal MV, van Abeelen AF, Painter RC, van der Postal service JA, Roseboom TJ. Consequences of hyperemesis gravidarum for offspring: a systematic review and meta-analysis. BJOG. 2011;118:1302–13.
-
Asker C, Norstedt Wikner B, Källén B. Use of antiemetic drugs during pregnancy in Sweden. Eur J Clin Pharmacol. 2005;61:899–906.
-
Källén B, Mottet I. Delivery outcome later on the utilise of meclozine in early pregnancy. Eur J Epidemiol. 2003;18:665–9.
-
Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. North Engl J Med. 2013;368:814–23.
-
Matok I, Gorodischer R, Koren Thou, Sheiner E, Wiznitzer A, Levy A. The safety of metoclopramide utilise in the first trimester of pregnancy. N Engl J Med. 2009;360:2528–35.
-
Sørensen HT, Nielsen GL, Christensen K, Tage-Jensen U, Ekbom A, Baron J. Birth outcome following maternal apply of metoclopramide. The Euromap study group. Br J Clin Pharmacol. 2000;49:264–8.
-
Bird AL, Grant CC, Bandara DK, Mohal J, Atatoa-Carr PE, Wise MR, Inskip H, Miyahara M, Morton SM. Maternal health in pregnancy and associations with adverse nascency outcomes: prove from growing up in New Zealand. Aust North Z J Obstet Gynaecol. 2017;57:xvi–24.
-
Petry CJ, Seear RV, Wingate DL, Manico L, Acerini CL, Ong KK, Hughes IA, Dunger DB. Associations betwixt paternally transmitted fetal IGF2 variants and maternal circulating glucose concentrations in pregnancy. Diabetes. 2011;60:3090–6.
-
Petry CJ, Mooslehner K, Prentice P, Hayes MG, Nodzenski K, Scholtens DM, Hughes IA, Acerini CL, Ong KK, Lowe WL Jr, Dunger DB. Associations betwixt a fetal imprinted gene allele score and late pregnancy maternal glucose concentrations. Diabetes Metab. 2017;43:323–31.
-
Petry CJ, Sanz Marcos N, Pimentel G, Hayes MG, Nodzenski 1000, Scholtens DM, Hughes IA, Acerini CL, Ong KK, Lowe WL Jr, Dunger DB. Associations betwixt fetal imprinted genes and maternal blood pressure in pregnancy. Hypertension. 2016;68:1459–66.
-
Fisher BG, Thankamony A, Hughes IA, Ong KK, Dunger DB, Acerini CL. Prenatal paracetamol exposure is associated with shorter anogenital distance in male infants. Hum Reprod. 2016;31:2642–l.
-
Phung H, Bauman A, Nguyen TV, Young L, Tran Thousand, Hillman K. Take chances factors for depression birth weight in a socio-economically disadvantaged population: parity, marital status, ethnicity and cigarette smoking. Eur J Epidemiol. 2003;18:235–43.
-
Bolin Grand, Åkerud H, Cnattingius S, Stephansson O, Wikström AK. Hyperemesis gravidarum and risks of placental dysfunction disorders: a population-based cohort report. BJOG. 2013;120:541–7.
-
Roseboom TJ, Ravelli AC, van der Post JA, Painter RC. Maternal characteristics largely explain poor pregnancy effect after hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 2011;156:56–9.
-
Vlachodimitropoulou Koumoutsea E, Gosh S, Manmatharajah B, Ray A, Igwe-Omoke Northward, Yoong W. Pregnancy outcomes in severe hyperemesis gravidarum in a multi-ethnic population. J Obstet Gynaecol. 2013;33:455–viii.
-
Hastoy A, Lien Tran P, Lakestani O, Barau K, Gérardin P, Boukerrou M. Hyperemesis gravidarum and pregnancy outcomes. J Gynecol Obstet Biol Reprod. 2015;44:154–63.
-
Buyukkayaci Duman N, Ozcan O, Bostanci MÖ. Hyperemesis gravidarum affects maternal sanity, thyroid hormones and fetal health: a prospective case command study. Arch Gynecol Obstet. 2015;292:307–12.
-
Kuru O, Sen South, Akbayır O, Goksedef BP, Ozsürmeli Thousand, Attar E, Saygılı H. Outcomes of pregnancies complicated past hyperemesis gravidarum. Arch Gynecol Obstet. 2012;285:1517–21.
-
Vikanes ÅV, Støer NC, Magnus P, Grjibovski AM. Hyperemesis gravidarum and pregnancy outcomes in the Norwegian female parent and child cohort - a cohort written report. BMC Pregnancy Childbirth. 2013;13:169.
-
Behrman CA, Hediger ML, Scholl TO, Arkangel CM. Nausea and vomiting during teenage pregnancy: furnishings on birth weight. J Adolesc Health Care. 1990;11:418–22.
-
Temming L, Franco A, Istwan Northward, Rhea D, Desch C, Stanziano G, Joy S. Agin pregnancy outcomes in women with nausea and vomiting of pregnancy. J Matern Fetal Neonatal Med. 2014;27:84–8.
-
Zhou Q, O'Brien B, Relyea J. Severity of nausea and vomiting during pregnancy: what does it predict? Nativity. 1999;26:108–14.
-
Czeizel AE, Puhó E. Association betwixt astringent nausea and vomiting in pregnancy and lower rate of preterm births. Paediatr Perinat Epidemiol. 2004;18:253–9.
-
Zhang J, Cai WW. Severe vomiting during pregnancy: antenatal correlates and fetal outcomes. Epidemiology. 1991;2:454–seven.
-
Weigel MM, Reyes Yard, Caiza ME, Tello N, Castro NP, Cespedes S, Duchicela S, Betancourt Thou. Is the nausea and vomiting of early pregnancy actually feto-protective? J Perinat Med. 2006;34:115–22.
-
Koren G, Madjunkova South, Maltepe C. The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome - a systematic review. Reprod Toxicol. 2014;47:77–80.
-
Berkovitch K, Mazzota P, Greenberg R, Elbirt D, Addis A, Schuler-Faccini 50, Merlob P, Arnon J, Stahl B, Magee L, Moretti Chiliad, Ornoy A. Metoclopramide for nausea and vomiting of pregnancy: a prospective multicenter international study. Am J Perinatol. 2002;19:311–6.
-
Chortatos A, Haugen M, Iversen PO, Vikanes Å, Eberhard-Gran Yard, Bjelland EK, Magnus P, Veierød MB. Pregnancy complications and birth outcomes among women experiencing nausea just or nausea and vomiting during pregnancy in the Norwegian mother and child accomplice study. BMC Pregnancy Childbirth. 2015;xv:138.
-
Colodro-Conde L, Cross SM, Lind PA, Painter JN, Gunst A, Jern P, Johansson A, Lund Maegbaek G, Munk-Olsen T, Nyholt DR, Ordoñana JR, Paternoster L, Sánchez-Romera JF, Wright MJ, Medland SE. Cohort profile: nausea and vomiting during pregnancy genetics consortium (NVP genetics consortium). Int J Epidemiol. 2017;46:e17.
-
Fejzo MS, Sazonova OV, Sathirapongsasuti JF, Hallgrímsdóttir IB, 23andMe Research Team, Vacic V, MacGibbon KW, Schoenberg FP, Mancuso N, Slamon DJ, Mullin PM. Placenta and ambition genes GDF15 and IGFBP7 are associated with hyperemesis gravidarum. Nature Comm. 2018; doi: https://doi.org/10.1038/s41467-018-03258-0.
-
Sherman Pow, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol. 2002;186(5 Suppl Understanding):S190–seven.
-
Crozier SR, Inskip HM, Godfrey KM, Cooper C, Robinson SM, SWS Study Grouping. Nausea and vomiting in early pregnancy: effects on food intake and diet quality. Matern Child Nutr. 2017;xiii:e12389.
-
Koren G, Madjunkova S, Maltepe C. The protective effects of nausea and vomiting of pregnancy against adverse fetal event--a systematic review. Reprod Toxicol. 2014;47:77–80.
-
Furneaux EC, Langley-Evans AJ, Langley-Evans SC. Nausea and vomiting of pregnancy: endocrine footing and contribution to pregnancy result. Obstet Gynecol Surv. 2001;56:775–82.
-
Askling J, Erlandsson Thou, Kaijser M, Akre O, Ekbom A. Sickness in pregnancy and sexual practice of kid. Lancet. 1999;354:2053.
-
Petry CJ, Ong KK, Burling KA, Barker P, Perry JRB, Acerini CL, Hughes IA, Dunger DB, O'Rahilly S. GDF15 concentrations in maternal serum associated with airsickness in pregnancy: the Cambridge baby growth study. BioRXIV. 2017; https://doi.org/ten.1101/221267.
-
O'Rahilly South. GDF15-from biomarker to allostatic hormone. Prison cell Metab. 2017;26:807–viii.
-
Pisa Fe, Casetta A, Clagnan E, Michelesio Due east, Vecchi Brumatti L, Barbone F. Medication use during pregnancy, gestational historic period and engagement of delivery: understanding between maternal cocky-reports and health database data in a cohort. BMC Pregnancy Childbirth. 2015;fifteen:310.
Acknowledgements
We give thanks all the families that took part in the Cambridge Baby Growth Study, and we acknowledge the crucial function played by the research nurses specially Suzanne Smith, Ann-Marie Wardell and Karen Forbes, staff at the Addenbrooke's Wellcome Trust Clinical Enquiry Facility, and midwives at the Rosie Motherhood Hospital.
Funding
Funding for this study has come up from the Wellbeing of Women (the Royal Higher of Obstetricians and Gynaecologists, UK) (RG1644). Other core funding has come up from the Medical Research Quango (7500001180), European Matrimony Framework 5 (QLK4-1999-01422), the Mothercare Charitable Foundation (RG54608), Newlife Foundation for Disabled Children (07/20), and the World Cancer Enquiry Fund International (2004/03). In improver, there has been support from National Institute for Wellness Research Cambridge Biomedical Research Centre. KO is supported by the Medical Research Council (Unit Programme number: MC_UU_12015/2). The sponsors did not take any role in the study design, in the collection, analysis or the estimation of the data, in the writing of the manuscript or in the decision to submit it for publication.
Availability of data and materials
All data generated or analysed during this report are included in this published article [and its Additional file 1].
Writer data
Affiliations
Contributions
CP designed and analysed the data for this specific written report and drafted the manuscript with KB and DD. KO, IH, CA and DD designed, established and oversee the Cambridge Infant Growth Study. All authors commented on the draft manuscript and read and approved the final manuscript.
Corresponding writer
Ethics declarations
Ethics approval and consent to participate
The Cambridge Infant Growth Written report was canonical by the Cambridge Local Research Ideals Committee, Addenbrooke's NHS Trust, Addenbrooke's Hospital, Cambridge, U.k. (Ref.: LREC 00/325). All procedures followed were in accordance with the institutional guidelines. Written informed consent was obtained from all the study participants.
Competing interests
The authors declare that they have no competing interests.
Publisher'south Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Boosted file
Additional file ane:
Description of information: the data that was associated with the manuscript entitled "Vomiting in pregnancy is associated with a higher risk of low birth weight: a cohort study" by Petry et al. (https://doi.org/ten.1186/s12884-018-1786-1) (XLSX 149 kb)
Rights and permissions
Open up Access This commodity is distributed under the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in whatever medium, provided you lot give appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/one.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
Virtually this commodity
Cite this commodity
Petry, C.J., Ong, K.Chiliad., Beardsall, K. et al. Airsickness in pregnancy is associated with a higher risk of low birth weight: a cohort study. BMC Pregnancy Childbirth 18, 133 (2018). https://doi.org/ten.1186/s12884-018-1786-ane
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12884-018-1786-1
Keywords
- Hyperemesis gravidarum
- Nausea
- Anti-emetic
- Small for gestational historic period
My Baby's Movements in Womb Make Me Vomit
Source: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-018-1786-1